Download this article and more from our free toolbox!
By Sean M. Scott & Briana C. Scott
Lessons Learned from the Notre Dame Cathedral
On April 15, 2019, fire broke out beneath the roof of the Notre-Dame de Paris Cathedral in Paris. Over 400 firefighters fought the blaze, which grew uncontrollably and consumed approximately two thirds of the roof structure, including the 300-foot wooden spire. The spire ultimately crashed through the roof, bringing down with it stone, stained glass windows, and the handcrafted, vaulted oak ceiling dating back to the 13th century. The initial damage assessments for the restoration of the cathedral and many of its priceless artifacts range from $790 million to $1 billion.
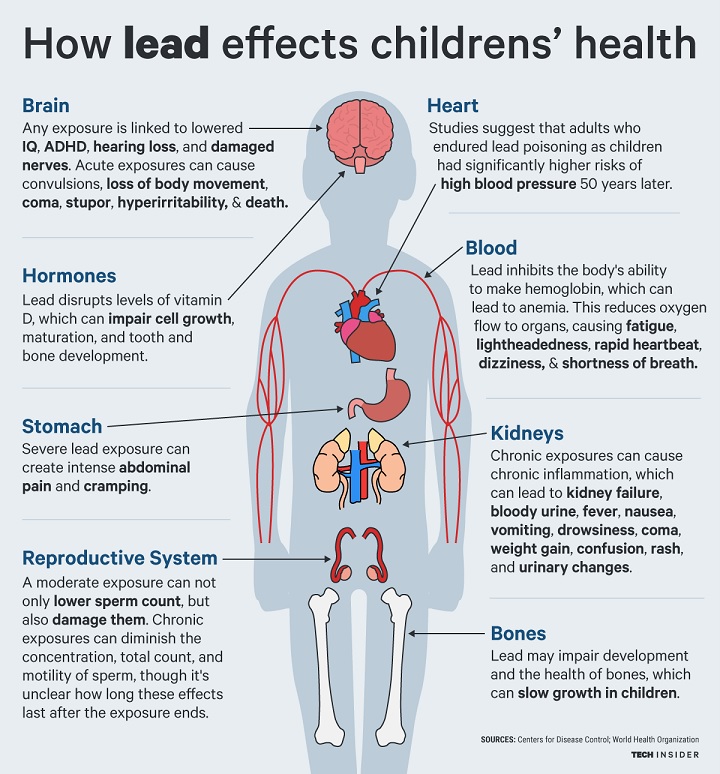
The roof’s various layers and spire, composed of approximately 450 tons of lead, quickly melted as the fire temperatures exceeded 1,400° F. As the smoke’s distinct yellow tinge suggested, the lead vaporized and created a toxic fallout of lead dust that was deposited across Paris.
As a result, all workers involved in the restoration are required to wear protective hazardous material suits and respirators. They also must regularly take blood tests for lead exposure, as lead levels at the site were found to far exceed the recommended limits set by French health authorities.
An Unexpected Threat – Lead Fumes and Fallout
In July of 2019, reports began to emerge of an unexpected threat to residents in the vicinity of the cathedral: lead poisoning. According to French media, areas close to the cathedral have levels of lead ranging between 500 and 800 times the official safe level. [1]
During a structure fire, lead fumes are produced when lead or lead-containing materials are heated to temperatures above 932° F. At these temperatures, lead vapor is released in the form of highly toxic lead oxide fumes. This vapor then condenses into solid fume particles which are released into the atmosphere. [2] This is one of the reasons why U.S. Federal law prohibits the use of heat guns that operate above 1100°F to strip lead based paint. (Code of Federal Regulations 24 § 35.140.)
Lead oxide is highly soluble in body fluids. The particle size of the metal fumes range between 0.1-0.7 microns, which increases the likelihood of inhalation and deposition of the fume directly into the bloodstream. When materials containing lead vaporize in a structure fire, the lead-laden smoke and other combustion byproducts combine to form toxic particulate matter that later permeates surfaces in the form of ultra-fine lead dust. Molten lead or lead fumes may also contain other toxic byproducts including chromium, cobalt, arsenic, selenium, cadmium, antimony, and mercury, as occurring in emissions from metal production. All of these metals and chemicals are listed in the Clean Air Act (CAA Title III) as being hazardous air pollutants (HAPs) that should be subjected to testing.[3]

Photo Courtesy of Wikimedia Commons
What is Lead?
The World Health Organization states that lead is an odorless heavy metal with a bluish-grey color. It has a low melting point, is easily molded and shaped, and can be combined with other metals to form alloys. Lead is non-biodegradable, and constitutes 0.002% of the Earth’s crust, and in nature exists mainly as lead sulphide.
The toxic nature of lead has been known since at least 2000 BC. Lead poisoning was common in Roman times, due to the use of lead in water pipes, earthenware containers and wine storage vessels. It is even believed to be primarily responsible for the collapse of the Roman Empire. Lead acetate was used in a syrup known as sapa, which served as a sweetener of wine. Its prolonged use was considered to have caused dementia to many Roman emperors. Lead poisoning associated with occupational exposure was first reported in 370 BC. In 1767, Benjamin Franklin obtained a list of patients in La Charité Hospital in Paris who had been admitted because of symptoms which, although not recognized as such at the time, were evidently those of lead poisoning. All the patients were engaged in occupations that exposed them to lead. Lead poisoning became common among industrial workers in the 19th and early 20th centuries, when workers were exposed to the substance while engaged in trades such as smelting, painting, plumbing, and printing. [4]
A Hidden Threat Revealed – Lead Contamination in New Buildings
Although the sheer volume of lead that vaporized in the Notre Dame fire was unique, fire restoration practitioners need to be aware of the likelihood that lead may be present in any fire damaged structure, especially those built after 1978. Now you may be asking yourself, “did he just say after 1978?” Believe it or not, lead based products are still being used in homes and buildings and are imported into the United States in varying forms to this day. [5] It is conceivable that the level of lead contamination resulting from molten lead and lead-laden fumes could pose a greater risk of exposure, far above the relatively small amount found in lead-based paint or ceramic tile glazing. This could come from melted fishing weights, plastics such as polyvinyl chloride (PVC), ammunition, automotive batteries, and a wide range of common household products.

A garage that was destroyed by fire. Numerous items and materials here likely contain lead.

The above photograph is another garage that was gutted by fire. Although this home was built well after 1978, there are numerous materials and items here that are known to contain lead. Fires at this level of damage should be tested for lead contamination as well as the presence of other toxic substances.
Although the Federal government banned the manufacture of lead-based paint in 1978, stockpiles of the paint were still sold to the public until the supplies were exhausted. As of 2006, an estimated 22% of U.S. homes (23.2 million) still contained lead-based paint hazards. [6]
Paint or other surface coatings that contain lead include chrome yellow (lead(II) chromate), red lead (lead(II,IV) oxide), and white lead (lead(II) carbonate). These are the most common forms of lead-based paint equal to or exceeding 1.0 milligram per square centimeter, or 0.5 percent by weight, or 5,000 parts per million by weight.
Here are some common building materials and household items that may contain lead.
Building Products:
 Lead-based paints
Lead-based paints- Ceramic tile glazing
- Porcelain glazing on bathtubs and sinks
- Stained glass windows
- Lead water piping
- Copper water pipe solder
- Lead used for cast iron pipe connections (lead & oakum seals)
- Roof flashings
- Lead lag shields used as wall or sill plate anchors
- Alarm system back-up batteries
- Sink faucet sprayer weights
- PVC (Polyvinyl Chloride)
- Solar cells
- Lead used in galvanizing, annealing, and plating [7]
- Rubber and plastics (Lead oxide and lead chromate) [8]
Household Items:
 Diving weights
Diving weights- Fishing weights and lures
- Ammunition (shotgun shells, lead shot, pellets, bullets, recoil reducers)
- Automotive batteries
- Tire balance weights
- Electronics
- Crystal glassware
- Jewelry
- Antique figurines
- Older earthenware, bone china and porcelain (especially from Mexico and South America)
- Cosmetics and hair dye products (Lead acetate)
- Artificial turf [9]
- Artificial Christmas trees [10]
- Drapery or curtain weights
- Children’s toys
- Children’s books printed prior to 1985 (lead used in ink for illustrations)
- Colored zippers (lead-based paint)
- Clothing snap closures (jackets, pants, overalls, etc.) Visit the U.S. Consumer Product Safety Commission for more details at: https://www.cpsc.gov/
The Dangers of Lead Exposure to Workers
The current Permissible Exposure Limit (PEL) set by OSHA is 50 micrograms of lead per cubic meter of air (50 ug/m^3), averaged over an 8-hour workday. One microgram is equal to one millionth (1×10−6) of a gram.
Although OSHA has set a PEL for lead, the World Health Organization, along with numerous other organizations and government agencies, have stated that “There is no known level of lead exposure that is considered safe.”[11,12,13,14,15]
Environmental exposure to lead can be absorbed into the body through inhalation (with 30% to 50% of the inhaled dose absorbed into the bloodstream), ingestion (with 8% to 15% of the ingested dose absorbed into the bloodstream) and, to a limited extent, dermal contact. [16]
OSHA Regulation 1910.1025 App A – Substance Data Sheet for Occupational Exposure to Lead, states that lead can be absorbed into your body by inhalation (breathing) and ingestion (eating). When lead is scattered in the air as a dust, fume, or mist, it can be inhaled and absorbed through your lungs and upper respiratory tract. Inhalation of airborne lead is generally the most prevalent source of occupational lead absorption.
A significant portion of the lead that you inhale or ingest will enter your bloodstream. Once in your bloodstream, it is circulated throughout your body and stored in various organs and body tissues. Some of this lead is quickly filtered out of your body and excreted, but some remains in the blood and tissues.
Even though you may not be aware of any immediate symptoms of disease, this lead stored in your tissues can be slowly causing irreversible damage: first to individual cells, then to your organs and whole-body systems.
Effects of overexposure to lead – Short term (acute) overexposure.
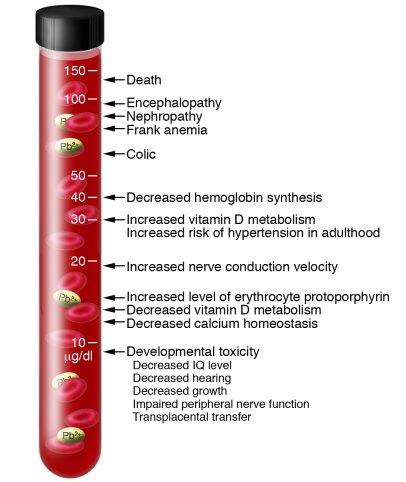
Lead is a potent, systemic poison that serves no known useful function once absorbed by your body. Taken in large enough doses, it can become fatal in a matter of days. A condition called acute encephalopathy may arise which develops quickly to seizures, coma, and death from cardiorespiratory arrest. Encephalopathy is a term that means brain disease, damage, or malfunction. It can present a broad spectrum of symptoms that range from mild, such as some memory loss or subtle personality changes, to severe, such as dementia, seizures, coma, or death. [17]
Similar forms of encephalopathy may also arise from extended, chronic exposure to lower doses of lead. There is no sharp dividing line between rapidly developing acute effects of lead, and chronic effects which take longer to acquire.
Long-term (chronic) overexposure.
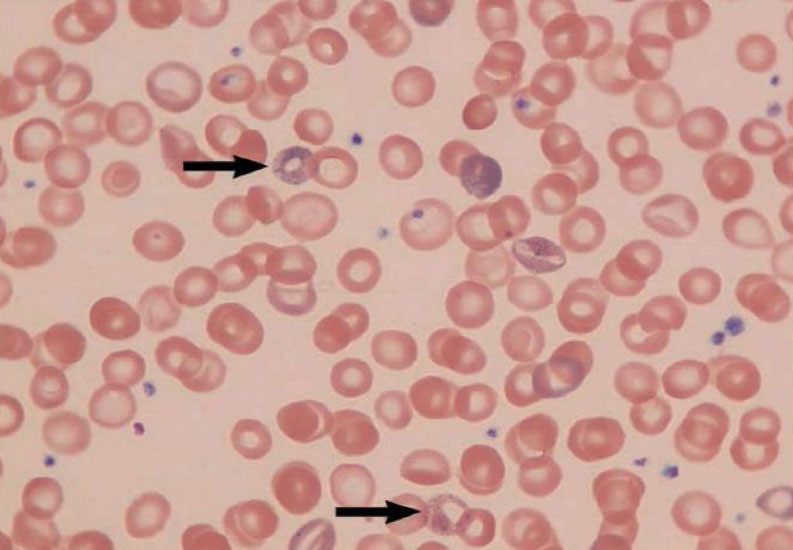
Chronic overexposure to lead may result in severe damage to the nervous, urinary, and reproductive systems. In lead colic there may be severe abdominal pain.
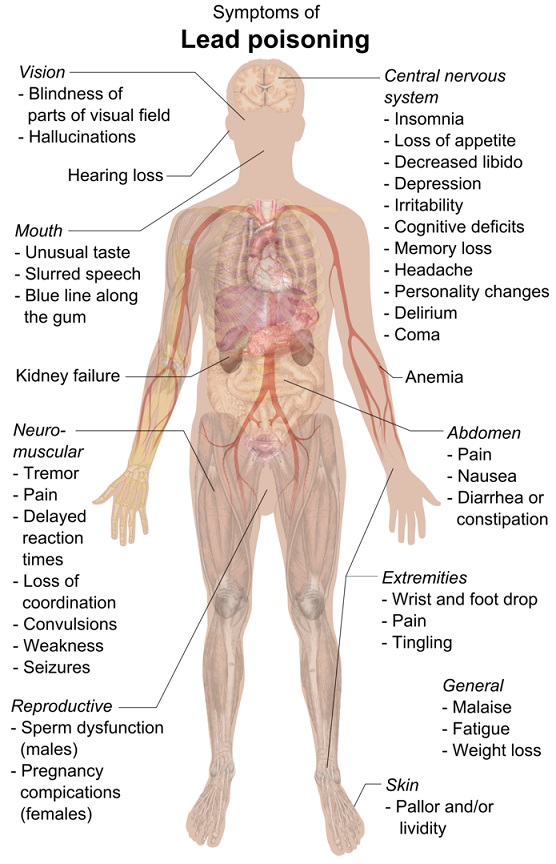
By Mikael Häggström – Own work, CC0, https://commons.wikimedia.org/w/index.php?curid=40804069
Damage to the central nervous system in general and the brain (encephalopathy) in particular is one of the most severe forms of lead poisoning. The most severe, often fatal, form of encephalopathy may be preceded by vomiting, a feeling of dullness progressing to drowsiness and stupor, poor memory, restlessness, irritability, tremors, and convulsions. It may arise suddenly with the onset of seizures, followed by coma, and death.
There is a tendency for muscular weakness to develop at the same time. This weakness may progress to paralysis often observed as a characteristic “wrist drop” or “foot drop”. This is a manifestation of a disease of the nervous system called peripheral neuropathy. Chronic overexposure to lead also results in kidney disease with few, if any, symptoms appearing until extensive and most likely permanent kidney damage has occurred. Routine laboratory tests reveal the presence of this kidney disease only after about two-thirds of kidney function is lost. When overt symptoms of urinary dysfunction arise, it is often too late to correct or prevent worsening conditions, and progression to kidney dialysis or death is possible. Chronic overexposure to lead impairs the reproductive systems of both men and women. This may result in decreased sex drive, impotence and sterility in men. It can also alter the structure of sperm cells, raising the risk of birth defects. There is evidence of miscarriage and stillbirth in women whose husbands were exposed to lead or who were exposed to lead themselves. Lead exposure also may result in decreased fertility, and abnormal menstrual cycles in women.
The course of pregnancy may be adversely affected by exposure since lead crosses the placental barrier and poses risks to developing fetuses. Children born of parents either one of whom were exposed to excess lead levels are more likely to have birth defects, mental retardation, behavioral disorders or die during the first year of childhood. [18]
Once your blood lead level climbs above 40 ug/100g, your risk of disease increases. An individual’s response to lead can widely vary, thus it is difficult to say that a particular lead blood level (PbB) in a given person will cause a particular effect. Studies have associated fatal encephalopathy with PbBs as low as 150 ug/100g. Other studies have shown different forms of disease in workers with PbBs well below 80 ug/100g. Your PbB is a crucial indicator of the risks to your health, but one other factor is also extremely important: the length of time you have had elevated PbBs. The longer you have an elevated PbB, the greater the risk that large quantities of lead are being gradually stored in your organs and tissues (body burden). The greater your overall body burden, the greater the chances of substantial permanent damage. Some studies indicate that lead can remain in your bones for decades. [18]
The Centers for Disease Control has conducted studies that found lead can be absorbed through the skin. If you handle lead and then touch your eyes, nose, or mouth, you could be exposed. Lead dust can also get on your clothes and hair. If this happens, it’s possible that you may track home some of the lead dust, which may also expose your family. This is called “take home” lead, and it can harm anyone who comes in contact with it. [19,20]
Carcinogenicity of Lead
Lead and lead compounds are reasonably anticipated to be human carcinogens based on limited evidence of carcinogenicity from studies in humans and sufficient evidence of carcinogenicity from studies in experimental animals.
Lead exposure has been associated with increased risk of lung, stomach, and urinary-bladder cancer in diverse human populations. However, most studies of lead exposure and cancer reviewed had limitations, including poor exposure assessment and failure to control for confounding by other factors that could increase the risk of cancer (such as lifestyle factors and concurrent occupational exposure to other carcinogens), and did not demonstrate relationships between the level or duration of exposure and the magnitude of cancer risk. The crude exposure measures used in most studies, such as treating whole plants or occupations as having uniform exposure, may have limited the magnitude of risk estimates, most of which were modest.
Evidence from epidemiological studies is compatible with small increases in the risk of lung or stomach cancer; however, this evidence must be weighed against the potential for confounding by factors such as smoking, diet, or co-exposure to arsenic. [21]
The Dangers of Lead Exposure – Workers’ Families and Children
 Lead poses the greatest risks to young children, infants, and fetuses because the physical and behavioral effects of exposure occur at lower exposure levels in children than in adults. A dose of lead that may have little effect on an adult can have a significant effect on a child. In children, low levels of lead exposure have been linked to damage to the central and peripheral nervous system, learning disabilities, shorter stature, impaired hearing, and impaired formation and function of blood cells. [22,23,24]
Lead poses the greatest risks to young children, infants, and fetuses because the physical and behavioral effects of exposure occur at lower exposure levels in children than in adults. A dose of lead that may have little effect on an adult can have a significant effect on a child. In children, low levels of lead exposure have been linked to damage to the central and peripheral nervous system, learning disabilities, shorter stature, impaired hearing, and impaired formation and function of blood cells. [22,23,24]
No safe blood lead level in children has been identified. Even low levels in the blood have been shown to affect IQ, ability to pay attention, and academic achievement. Effects of this exposure cannot be corrected. The CDC now uses a blood lead reference value of 5 micrograms per deciliter to identify children with blood lead levels that are much higher than average. Until 2012, children were identified as having a blood lead “level of concern” if the test result was 10 or more micrograms per deciliter. [25]
Key Units of Measurement
 μg (Microgram): A microgram is 1/1000th of a milligram (or one millionth of a gram). To put this unit into perspective, a penny weighs 2 grams. To get a microgram, you would need to divide the penny into 2 million pieces.
μg (Microgram): A microgram is 1/1000th of a milligram (or one millionth of a gram). To put this unit into perspective, a penny weighs 2 grams. To get a microgram, you would need to divide the penny into 2 million pieces.
ft² (Square foot): One square foot is equal to an area that has a length of one foot (12 inches) and a width of one foot (12 inches).
μg/dL: Micrograms per deciliter was formerly used to measure the level of lead in children’s blood to establish whether intervention is needed. A deciliter (1/10thof a liter) is a little less than half a cup.
μg/gram: Micrograms per gram of sample, equivalent to parts per million (ppm) by weight. Used to measure lead in soil.
μg/ft²: Micrograms per square foot are the units used to measure levels of lead in dust samples. The clearance report should have the dust sampling results listed in μg/ft² (micrograms per square foot).
mg/cm2: Milligrams per square centimeter. This unit is used to measure lead in paint.
percent: Percent by weight, usually used for lead-based paint (1 percent = 10,000 μg/gram)
ppm: Parts per million by weight, equivalent to μg/gram (10,000 ppm = 1 percent). It is used to measure lead in paint and soil.
U.S. Department of Housing and Urban Development (HUD) Lead-Based Paint Standards
HUD has set a standard for paint or other surface coatings as those that contain at least 1 milligram per centimeters squared (mg/cm2) of lead; 0.5 percent lead; or 5,000 parts per million lead by dry weight.[26]
The EPA Set New Standards
On June 21, 2019, the EPA announced new, tighter standards for lead in dust on floors and window sills in order to protect children from the harmful effects of lead exposure. The strengthened standards become effective 180 days after publication in the Federal Register. Under the 2001 dust-lead hazard standards, lead is considered a hazard when equal to or exceeding 40 micrograms (μg) of lead in dust per square foot (ft2) on floors, 250 micrograms of lead in dust per square foot on interior window sills, and 400 parts per million (ppm) in bare soil in children’s play areas or 1200 ppm average for bare soil in the rest of the yard. In addition, paint in deteriorating condition, on a friction or impact surface, or on certain chewable surfaces, is also defined as a hazard. When the 2019 final rule becomes effective, these standards will be lowered from 40 μg/ft2 and 250 μg/ft2 to 10 μg/ft2 and 100 μg/ft2 on floors and window sills, respectively.[27] Individual states can have more protective standards than federal OSHA, e.g., California.
Conclusions & Considerations
Historically, fire restoration practitioners rarely test for lead in fire or smoke damaged buildings constructed after 1978. The reason for this is mainly that testing for lead in newer buildings isn’t considered mandatory or customary. This is a gross oversight. The reality is that most buildings, even those built today, contain some level of lead in the building materials or contents, and in some cases, enough to pose serious health risks or even death to those exposed.
To illustrate how much of a concern lead in household products should be in comparison to lead-based paint, let’s look at the amounts of lead in three different items: a door painted with lead-based paint, a 6 oz. lead fishing weight, and a car battery. Now, let’s say each of these items were completely destroyed and/or vaporized in separate residential structure fires. (Lead-based paint is defined as any paint that has at least 1 milligram of lead per square centimeter (mg/cm^2) or 0.5% by dry weight (5,000 micrograms per gram dry weight, or 5,000 parts per million).

For the purpose of this example, a door takes about a pint of paint to cover both sides. A pint of lead-based paint contains approximately 1/10th of an ounce of lead, or 2,834.95 milligrams.
A 6 oz. fishing weight contains 170,094 milligrams of lead. (28,349.52 milligrams per oz. x 6 oz. = 170,094 milligrams).
A typical car battery contains approximately 21 lbs. of lead or 9,525,264 milligrams of lead. (28,349.52 milligrams of lead per oz. x 16 oz.’s per lb. x 21 lbs. = 9,525,438.72 milligrams).
The current Permissible Exposure Limit (PEL) set by OSHA is 50 micrograms of lead per cubic meter of air (50 ug/m^3), averaged over an 8-hour workday. (NOTE: 1 milligram is equal to 1,000 micrograms).
Given these examples, doesn’t it seem reasonable that structure fires occurring in homes or buildings built after 1978 could contain extremely hazardous levels of lead, even to the point of being immediately dangerous to life and health? Shouldn’t every structure fire that is suspected of containing lead products be tested? If not, how can anyone know whether or not the substance might be present?
How can an employer know whether his or her employees are being exposed to lead levels greater than 50 micrograms of lead per cubic meter of air averaged over an 8-hour workday?
Next, take into consideration the amount of lead dust that may be generated when entire neighborhoods are consumed by wildfires. Rarely will testing be conducted for lead or other toxic heavy metals in smoke damaged homes surrounding wildfire areas. At best, smoke damaged homes are only tested for the presence of soot, char, or ash, but not their chemical composition.
Restorers should be far more concerned about lead contamination from vaporized household products. Here are some suggestions:
- Treat every fire as though it has the potential of being contaminated by lead, regardless of age, and test for lead as soon as possible to avoid accidental exposure.
- All persons who need to enter a fire damaged structure (insurance adjusters, estimators, investigators, inventory specialists, laborers, occupants, etc.) should always wear proper personal protective equipment until the work area is tested and deemed lead-free.
- Perform a visual assessment of interior and exterior surfaces and contents to identify specific items or conditions that may be potential lead hazards.
- Have a certified environmental testing company take samples of the dust, soot, or ash to see if lead contaminants are present. If lead is found, the testing agency should provide a lead remediation protocol. No work should be done in or around the contaminated areas until the hazards have been abated by a state certified lead abatement contractor.
- Get a clearance test from an independent third-party laboratory after the lead is abated. This is used to verify if the abatement was done properly and provides all interested parties with written proof that the lead contaminants have been properly removed.
- Where lead dust may have contaminated textiles or soft goods (clothes, bedding, draperies, rugs, etc.), have sample articles tested for lead prior to cleaning. The use of ozone, hydroxyls, cleansers, or deodorizers are not effective to remove lead dust or other heavy metals and should not be used. In my opinion, clothes, bedding, soft goods, or children’s toys should not be cleaned if they have come in contact with lead dust or other toxic heavy metals. These items should be treated as contaminated and properly disposed of. Children often chew on their toys, clothes, blankets, and put things in their mouths. This is how lead can be ingested and is the main cause of lead poisoning amongst children.
The restoration industry can no longer afford to keep its head in the sand regarding the dangers of lead exposure and other toxic substances that are present in structure fire settings. Every fire is unique, and in severe fire settings, what may have combusted is often unknown. Protect yourself and others by testing for lead, heavy metals, and toxic VOC’s and wear proper personal protective equipment.
Your life, your colleagues’, workers’, and clients’ lives could depend on it.
For more information on testing for lead or other hazardous substances, contact Bruce Rosenblatt with Rarefied Air Environmental at 619-888-4840 or visit: http://rarefiedairenvironmental.com
SOURCES:
- Wired: The Notre Dame Fire Spread Toxic Lead Dust Over Paris https://www.wired.com/story/the-notre-dame-fire-spread-toxic-lead-dust-over-paris/?verso=true
- Ontario Ministry of Labor – Controlling the Lead Hazard https://www.labour.gov.on.ca/english/hs/pubs/lead/gl_lead_4.php and https://www.teck.com/media/2015-Products-Lead_Metal_SDS-T2.5.pdf
- EPA – Miscellaneous Lead Products https://www3.epa.gov/ttnchie1/ap42/ch12/final/c12s17.pdf
- WHO – Childhood Lead Poisoning https://www.who.int/ceh/publications/leadguidance.pdf
- CDC – Lead inhttps://www3.epa.gov/ttnchie1/ap42/ch12/final/c12s17.pdf Consumer Products https://www.cdc.gov/nceh/lead/prevention/sources/consumer-products.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fnceh%2Flead%2Ftips%2Ftoys.htm
- HUD – American Healthy Homes Survey – Lead and Arsenic Findings https://www.hud.gov/sites/documents/AHHS_REPORT.PDF
- EPA – Miscellaneous Lead Products https://www3.epa.gov/ttnchie1/ap42/ch12/final/c12s17.pdf
- Uhttps://www.hud.gov/sites/documents/AHHS_REPORT.PDF.S. Dept. of Health & Human Services – Report on Carcinogens, Fourteenth Edition – Lead and Lead Compounds https://ntp.niehs.nih.gov/ntp/roc/content/profiles/lead.pdf
- CDC – Childhood Lead Poisoning Prevention – Artificial Turf https://www.cdc.gov/nceh/lead/prevention/artificialturf.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fnceh%2Flead%2Ftips%2Fartificialturf.htm
- RTK Environmental Group https://rtkenvironmental.com/lead/warning-hidden-health-hazard-artificial-christmas-trees/
- WHO – Lead Poisoning & Health https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health
- PAHO – Lead Contamination https://www.paho.org/hq/index.php?option=com_content&view=article&id=8206:2013-lead-contamination&Itemid=39800&lang=en
- Florida Dept. of Health – Adult Lead Poisoning https://www.floridahealth.gov/environmental-health/lead-poisoning/adults.html
- Colorado Dept. of Public Health &Environment – Occupational Lead Exposure: Overview and Health Effects https://www.colorado.gov/pacific/sites/default/files/HHW_WS_Adult-Lead-Exposure-and-Health-Effects-Overview_1.pdf
- Laborer’s Health & Safety Fund – More Research Shows There’s No Safe Level of Lead Exposure https://www.lhsfna.org/index.cfm/lifelines/october-2018/more-research-shows-there-s-no-safe-level-of-lead-exposure/
- U.S. Dept. of Health & Human Services – Report on Carcinogens, Fourteenth Edition – Lead and Lead Compounds https://ntp.niehs.nih.gov/ntp/roc/content/profiles/lead.pdf
- Medicinenet.com – Encephalopathy facts https://www.medicinenet.com/encephalopathy/article.htm#what_causes_encephalopathy
- OSHA – Substance data sheet for occupational exposure to lead https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1025AppA
- NIOSH – LEAD Information for Workers https://www.cdc.gov/niosh/lead/?CDC_AAref_Val=https://www.cdc.gov/niosh/topics/lead/exposure.html
- New York State Dept. of Health – Health Dangers from Lead on the Job https://www.health.ny.gov/publications/2543/
- U.S. Dept. of Health & Human Services – Report on Carcinogens, Fourteenth Edition – Lead and Lead Compounds https://ntp.niehs.nih.gov/ntp/roc/content/profiles/lead.pdf
- Agency for Toxic Substances & Disease Registry – Lead Toxicity – Where Is Lead Found? https://www.atsdr.cdc.gov/csem/csem.asp?csem=34&po=5
- NIOSH – LEAD Information for Workers https://www.cdc.gov/niosh/lead/?CDC_AAref_Val=https://www.cdc.gov/niosh/topics/lead/exposure.html
- WHO – Childhood Lead Poisoning https://www.who.int/ceh/publications/leadguidance.pdf
- CDC – Blood Lead Levels in Children https://www.cdc.gov/nceh/lead/prevention/blood-lead-levels.htm
- HUD – “LEAD SPEAK” – A BRIEF GLOSSARY https://www.hud.gov/sites/documents/20264_LEADSPEAK.PDF
- EPA – Hazard Standards for Lead in Paint, Dust and Soil (TSCA Section 403) https://www.epa.gov/lead/hazard-standards-and-clearance-levels-lead-paint-dust-and-soil-tsca-sections-402-and-403
DISCLAIMER:
This paper is distributed as a public service for informational purposes only. While reasonable efforts were made to ensure the completeness and accuracy of its contents when published, no warranties or guarantees are made with respect to the completeness or accuracy of the information and opinions stated herein. The information and opinions stated herein may not be applicable to or suitable for every individual or situation and constitute the opinions of the Author and the sources of the information sited. Neither the Author nor Heritage Publishing & Communications, Ltd., shall be responsible to any user of the information for any injury, loss or damage of any kind or nature whatsoever that may be sustained as a consequence of the use and application of any information or opinions presented. In no event shall the Author nor Heritage Publishing & Communications, Ltd., be liable for any action taken or not taken by any person in reliance, directly or indirectly, upon the information and opinions provided herein. This publication is provided with the understanding that the Author, is not engaged in rendering legal advice and no legal opinions are provided by the Author. If legal or other professional advice or expert assistance is required, the services of a competent professional should be obtained.
All persons and entities using the information and opinions provided herein do so at their own risk and they hereby waive any and all claims against the Author and Heritage Publishing & Communications, Ltd. Such waiver includes any and all claims arising in contract or tort, and all forms of equitable relief and damages including without limitation compensatory, general, special, and consequential damages.
Without limiting any other disclaimer provided herein, under no circumstances shall the Author be liable for any injury, loss, or damage of any kind or nature whatsoever. Information in this newsletter relates to a subject that changes periodically due to changes in industry practices, science, and technology. All users of this paper acknowledge this disclaimer and agree to the limitations of liability stated above.
Do not use this information if you do not agree with this disclaimer and its limitations of liability as reasonable. If any portion of this disclaimer is found to be unenforceable under applicable law, the remainder of the disclaimer will remain enforceable.
ABOUT SEAN SCOTT:
 Mr. Scott is a licensed general contractor in the State of California who has spent over 39 years in the construction and restoration industry. As a second-generation fire and flood restoration contractor, he has been involved with literally thousands of property damage claims ranging from commercial and residential floods and fires, smoke claims, mold contaminations, subsidence and earthquake claims, explosions, vehicular collisions, and many other types of property damaging incidents. Throughout his career, Sean has worked with all the major insurance carriers that underwrite residential and commercial policies and has worked directly with claims adjusters, independent adjusters, third party administrators, public adjusters, and attorneys. Sean is also the author of two books, The Red Guide to Recovery – Resource Handbook for Disaster Survivors and Secrets of the Insurance Game and the co-author of a third book titled The Native Family Disaster Preparedness Handbook.
Mr. Scott is a licensed general contractor in the State of California who has spent over 39 years in the construction and restoration industry. As a second-generation fire and flood restoration contractor, he has been involved with literally thousands of property damage claims ranging from commercial and residential floods and fires, smoke claims, mold contaminations, subsidence and earthquake claims, explosions, vehicular collisions, and many other types of property damaging incidents. Throughout his career, Sean has worked with all the major insurance carriers that underwrite residential and commercial policies and has worked directly with claims adjusters, independent adjusters, third party administrators, public adjusters, and attorneys. Sean is also the author of two books, The Red Guide to Recovery – Resource Handbook for Disaster Survivors and Secrets of the Insurance Game and the co-author of a third book titled The Native Family Disaster Preparedness Handbook.
Sean has devoted his life to assisting individuals and families rebuild their homes, businesses, and lives and has witnessed first-hand the physical, emotional, and financial challenges people face once the first responders leave the scene. Since 2009, Sean’s award-winning book The Red Guide to Recovery has been adopted by fire departments, emergency management agencies, and relief organizations across the U.S.
Sean now uses his time and expertise to help people navigate the recovery process, speak on recovery and restoration topics, and consult with those in the restoration industry who need guidance to be more successful. He also provides expert witness testimony, estimating services on property damage claims, insurance claim appraisal and umpire services, construction defect investigation, and training.
For more information on disaster restoration and recovery, The Red Guide to Recovery – Resource Handbook for Disaster Survivors and Secrets of The Insurance Game both provide a wealth of insight and information on the recovery process.

For more information, contact Sean Scott
Office: 858-453-6767
Cell: 858-349-2262
Email: Sean@TheRedGuideToRecovery.com
Website: TheRedGuideToRecovery.com
©Copyright Heritage Publishing & Communications, Ltd. All Rights Reserved 2018